IVD STUDIES
As a specialized Contract Research Organization (CRO) in IVD studies (In Vitro Diagnostics), we provide comprehensive analytical and IVD clinical trials services, including analytical and clinical performance studies
Our services are designed to ensure that your device meets regulatory requirements from the U.S. Food and Drug Administration (FDA) and the European Union’s In Vitro Diagnostic Regulation (IVDR), as well as the Clinical and Laboratory Standards Institute (CLSI) standards, ISO 20916, ISO 14155, and other relevant requirements, including for example the WHO pre-qualification program.
All our services include regulatory affairs support and consulting on regulatory compliance to ensure complete, accurate, and compliant regulatory submissions. Our statistical analysis and documentation preparation for regulatory submissions are designed to meet FDA and IVDR requirements.
Contact us today to learn more about how our IVD Studies can help you meet regulatory requirements and achieve your research objectives.
Clinical Disciplines
- Clinical Chemistry
- Haematology, Haemostasis & Blood Transfusion
- Microbiology, Virology, Parasitology
- Histopathology
- Genetics
- And more…
Therapeutic areas
- Cardiovascular disease
- Precision Medicine & Oncology
- Infectious Diseases, Respiratory Diseases
- Neurological Disorders
- Rare Diseases
- Women’s Health
- And more…
BIOMARKERS, TECHNOLOGIES AND MORE
With extensive experience in conducting clinical research and IVD clinical trials across a range of therapeutic areas, our IVD CRO services are tailored to help you achieve your research objectives. Our team of experts will work with you to design and conduct clinical performance studies, manage and process samples, and provide data management and analysis.
We also offer Electronic Data Capture (EDC) system setup and support, Trial Master File (TMF) management, site qualification visits, and study monitoring.
Discover our range of biomarkers, technologies and applications for your IVD studies.
IVD biomarkers
- Infectious Diseases
- Physiological markers
- Cancer markers
- Companion Diagnostics
- Genetic Tests
- Auto-immunity
- Laboratory Developed Tests/LDTs
- And more…
Technologies & applications
- Immunoassays
- Next Generation Sequencing
- Multiplex
- Microfluidic Technology
- Molecular Diagnostics
- Near-Patient Tests / Point of Care Diagnostics
- Immunohistochemistry
- Lateral Flow technology
- Lay User Studies
- IVD Software

CONTACT US TODAY TO DISCUSS YOUR IVD PRODUCT NEEDS
IVD ANALYTICAL PERFORMANCE SERVICES
IVD Analytical Performance Services at MDx CRO
- Design and development of analytical performance studies in compliance with CLSI, FDA and IVDR and other technical standards.
- Data management and analysis using advanced statistical methods, ensuring the accuracy and completeness of study data
- Statistical analysis, providing reliable and accurate results
- Documentation preparation and protocol review for regulatory submissions, ensuring compliance with CLSI, FDA, and IVDR requirements
- Consulting on regulatory compliance helping clients navigate the complex regulatory landscape of IVD studies and ensuring compliance with CLSI, FDA, and IVDR requirements.

ANALYTICAL PERFORMANCE FAQs
-
What is IVD analytical performance?
IVD analytical performance is a critical component of the regulatory approval process for IVD devices, as it demonstrates the device’s ability to produce reliable and accurate results, which is essential for ensuring patient safety and effective clinical decision-making.
The European Union’s In Vitro Diagnostic Regulation (IVDR) and the U.S. Food and Drug Administration (FDA) both require IVD manufacturers to demonstrate the analytical performance of their devices as part of the regulatory submission process.
This typically involves conducting analytical performance studies to evaluate the device’s accuracy, precision, sensitivity, and specificity, as well as other performance characteristics.
-
What is the difference between analytical and clinical performance for IVDs?
Analytical performance refers to the ability of an IVD device to accurately measure the analyte(s) of interest in a clinical sample, while clinical performance refers to the ability of the device to accurately diagnose or predict the presence or absence of a specific medical condition or disease in patients.
While both types of performance are important for regulatory approval, clinical performance is particularly important for demonstrating the device’s ability to produce accurate and reliable results in a clinical setting.
IVD CLINICAL PERFORMANCE SERVICES
IVD Clinical Trials at MDx CRO
- Clinical Performance Study Protocol review
- Design and conduct of clinical performance IVD studies, in compliance with ISO 20916, ISO 14155, FDA, and IVDR requirements
- Sample management and processing, ensuring the highest quality of samples for accurate and reliable results
- Data management and analysis, using advanced statistical methods and tools to ensure accurate and reliable results
- Electronic Data Capture (EDC) system setup and support, allowing for efficient and secure data collection and management
- Trial Master File (TMF) management, ensuring the completeness and accuracy of essential study documents
- Site qualification visits, to ensure that study sites meet all regulatory and protocol requirements
- Study monitoring, to ensure that the study is conducted in compliance with GCP and study protocol requirements
- Regulatory affairs support, to ensure that all regulatory submissions are complete and accurate and meet all regulatory requirements
- Consulting on regulatory compliance, providing guidance on how to navigate the complex regulatory landscape of IVD clinical performance studies
- Statistical analysis, providing reliable and accurate results
- Documentation preparation for regulatory submissions, ensuring compliance with FDA and IVDR requirements
Evaluate your IVD CRO partner today with our fail-safe questionnaire!
| IVD CRO QUALIFICATION QUESTIONNAIRE | MDx |
|---|---|
| What services does the CRO provide? | |
| Clinical Operations | ✓ |
| Project Management, Medical Writing, Data Management, Statistics | ✓ |
| Regulatory Affairs | ✓ |
| Quality Assurance | ✓ |
| What devices are the CRO´s Subject Matter Experts in? | |
| Applications: Professional use, Point of Care, Self-Tests, Companion Diagnostics. All IVDR Classes. | ✓ |
| Technology: Immunoassays, NGS, Multiplex, Molecular, Lateral Flow, Microfluidic and others | ✓ |
| Therapeutic Areas: Infectious Diseases, Physiological Markers, Cancer Screening, Transfusion Medicine, Genetic Testing, Hematology, Hemostasis, Clinical Chemistry, Microbiology | ✓ |
| Software as a Medical Device/In Vitro Diagnostics | ✓ |
| Laboratory Developed Tests/ In-house developed tests | ✓ |
| Does the CRO have specific procedures for IVDs? | |
| For Clinical Operations following GCPs | ✓ |
| For Clinical Operations following GCPS, IVDR and ISO 20916 | ✓ |
| Is the CRO an Expert in the Following Regulations or Standards? | |
| GCPs | ✓ |
| GLPs | ✓ |
| IVDR | ✓ |
| ISO 20916 | ✓ |
| ISO 13485, ISO 15189 | ✓ |
| CLSI guidance, FDA requirements | ✓ |
| Where do the experts come from? | |
| IVD Notified Bodies | ✓ |
| Competent Authorities | ✓ |
| IVD Manufacturers | ✓ |
| IVD Auditors | ✓ |

CLINICAL PERFORMANCE FAQs
-
What is IVD clinical performance?
IVD clinical performance refers to the ability of an IVD device to accurately diagnose or predict the presence or absence of a specific medical condition or disease in patients. IVD clinical trials, with prospective or retrospective study designs are typically required to establish clinical performance.
This is a critical component of the regulatory approval process for IVD devices, as it demonstrates the device’s ability to produce reliable and accurate results, which is essential for ensuring patient safety and effective clinical decision-making.
-
Why is IVD clinical performance important?
IVD clinical performance is important because it demonstrates the device’s ability to produce reliable and accurate results, which is essential for ensuring patient safety and effective clinical decision-making.
Accurate and reliable diagnosis is critical for guiding patient treatment and management, and IVD devices play a key role in this process.
-
What factors are evaluated in IVD clinical performance studies?
IVD studies evaluate several factors, including diagnostic sensitivity, diagnostic specificity, positive predictive value, negative predictive value, and others.
These studies typically involve testing the device in a large and diverse patient population, including those with and without the target medical condition or disease.
-
What are the main gaps identified in IVD Clinical Performance Studies?
The main gap identified in IVD studies is the application of sources to demonstrate clinical performance. There is often a lack of clarity and consistency in the application of sources of clinical performance data, which can lead to confusion and inconsistencies in regulatory submissions.
Clinical performance data should be presented in a manner that is consistent with regulatory requirements, such as Annex XIII 2.3 of the IVDR. Clinical Performance studies are typically required unless the manufacturer can comfortably rely on other sources, including peer reviewed literature for example.
- Lack of consideration for professional, near-patient and self-test requirements: there is a lack of data and suitably designed studies that support clinical performance in different use settings, including professional, point of care and home-use settings.
- Insufficient clinical performance data for legacy devices: there is often insufficient clinical performance data available for legacy IVD devices, which can lead to concerns about the reliability and accuracy of the diagnostic testing.
- Insufficient sample size or patient population: some IVD clinical performance studies have insufficient sample size or patient population, which can result in inconclusive or unreliable study results.
- Incomplete data collection or analysis: notified Bodies and FDA often find identified instances where clinical performance study data collection or analysis is incomplete, which can lead to unreliable or inconclusive study results.
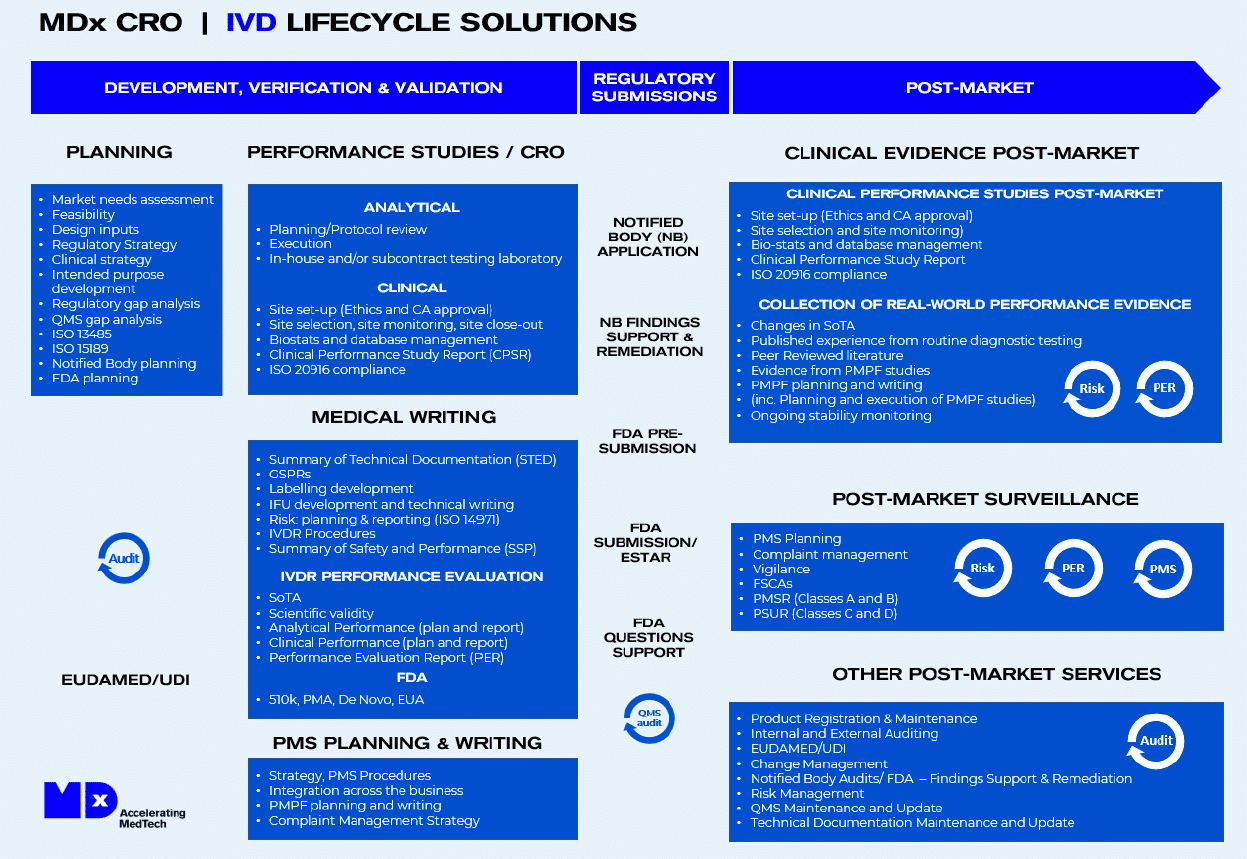
OUR IVD STUDIES & LIFECYCLE SOLUTIONS
At MDx CRO, we cover the full IVD lifecycle, from patent to IVD clinical trials, commercialization and beyond. We understand the complexities involved in bringing a new diagnostic test to market, and our team of experts has the experience and knowledge to guide you through every stage of the process.
Whether you need help with market research, product development, medical writing, regulatory compliance, or post-market support, we’ve got you covered. Trust us to be your partner throughout the entire diagnostics lifecycle, helping you to bring innovative and effective solutions to patients and healthcare providers.

MED IVD HEALTHTECH S.L ha sido beneficiaria de la subvención de contratos de trabajo de la Comunidad de Madrid, cofinanciado por el Fondo Social Europeo dentro de la Ayuda a la Recuperación para la Cohesión y los Territorios de Europa (REACT-UE), a través del Programa Operativo Regional FSE, en el marco del Programa Impulso a la Contratación Estable de Jóvenes para la Recuperación Económica, gestionado por la Dirección General del Servicio Público de empleo de la Consejería de Economía, hacienda y empleo de la Comunidad de Madrid.
© 2024 Copyright MDx | MedTech IVD CRO.
Dark mode is activated. Turn off