 March 9, 2023
March 9, 2023
In vitro diagnostic (IVD) devices are essential in healthcare as they provide accurate and reliable diagnostic information to healthcare providers. The development of an IVD device involves several stages, including research and development, design and prototyping, verification and validation, regulatory approval, and commercialization.
One of the critical steps in this process is the conduct of an IVDR clinical performance study to generate reliable and meaningful data to support regulatory approval and the device’s commercial success. In Europe, the in-vitro diagnostic regulation (EU IVDR) is now in force and all new products to market must meet very strict requirements of clinical performance.
The role of ISO 20916 in IVDR clinical performance studies
The design and execution of an IVD clinical performance study are critical to its success, and several factors must be considered to ensure that the study generates reliable and meaningful data. The International Organization for Standardization (ISO) has developed ISO 20916, a standard that provides guidance on the design and conduct of clinical studies for IVD medical devices. The standard is intended to help manufacturers, regulators, and other stakeholders ensure that IVD clinical performance studies are designed and conducted in a consistent and scientifically rigorous manner.
ISO 20916 covers several important aspects, including study design, sample size determination, selection of appropriate endpoints, statistical analysis, and reporting of study results. The standard emphasizes the importance of designing studies that are appropriate for the intended use of the IVD device and that incorporate good clinical practice (GCP) principles.
Both EU IVDR and ISO 20916 require a clear and well-defined protocol for the study, referred to in the regulation as the clinical performance study plan (CPSP).
The plan should specify the study objectives, inclusion and exclusion criteria for study participants, study endpoints, and statistical analysis plan, amongst many other requirements. It should also include procedures for data management and quality control to ensure the accuracy and reliability of the data collected.
Another important aspect of ISO 20916 is the requirement to ensure the safety and well-being of study participants. The standard emphasizes the importance of obtaining informed consent from study participants and protecting their privacy and confidentiality. The standard also requires that studies be conducted in compliance with ethical principles and regulatory requirements.
Alignment with EU IVDR
In addition to ISO 20916, the implementation of the EU IVDR has increased the importance of conducting IVD clinical performance studies as they are required for regulatory compliance. The IVDR replaced the previous In Vitro Diagnostic Directive (IVDD) and introduced more stringent requirements for IVD devices, including clinical evidence requirements. IVD manufacturers are now required to demonstrate clinical evidence that supports a device’s intended purpose and its’ safety and performance. This is particularly important, because insufficient clinical evidence could ultimately lead to a product refusal at the Notified Body resulting in additional costs and delays to bringing product to market.
Clinical performance studies represent the strongest level of evidence and they are usually expected by Notified Bodies, unless there is a justification for relying on other sources of clinical data.
Amongst many requirements, an IVDR clinical performance study is designed and conducted in compliance with GCP principles. ISO 20916 has additional requirements, and both the regulation and the standard should be considered by all diagnostic manufacturers when developing clinical performance study plans or protocols.
How can MDx CRO help?
MDx is a Medical Device & IVD Contract Research Organization (CRO) that can help IVD device manufacturers with their clinical performance studies by providing a range of services, including:
- study design
- site selection
- patient recruitment
- study monitoring
- data management
- statistical analysis
MDx has extensive experience in conducting clinical performance studies for IVD devices and a deep understanding of the regulatory requirements for these studies. Our team of professionals is well-trained and experienced in managing all aspects of the study, from protocol development to study execution and data analysis. We work closely with our clients to ensure that their studies are designed and conducted in compliance with applicable regulations and guidelines and that they generate reliable and meaningful data.
Evaluate your IVD CRO partner
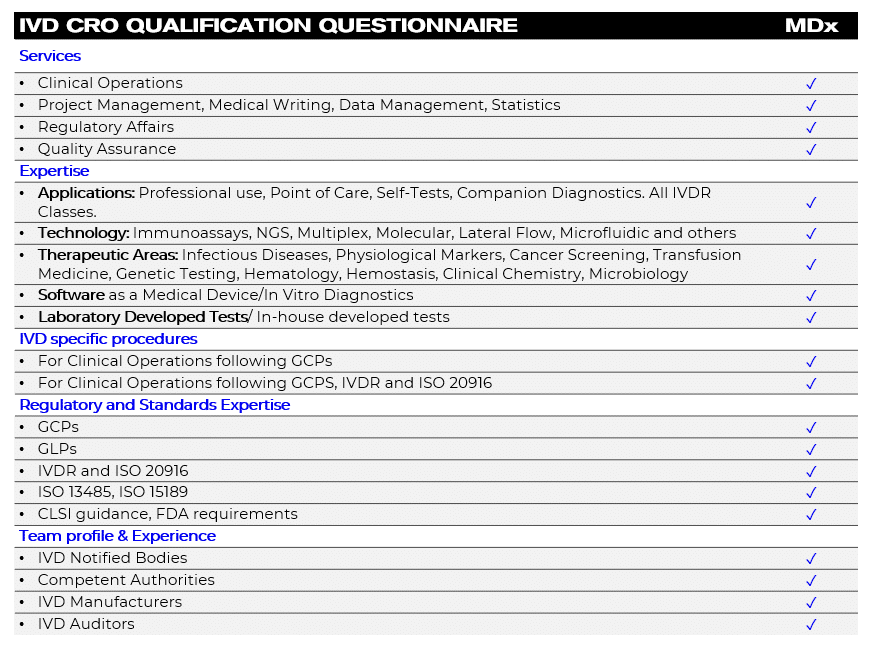 IVD CRO Qualification Questionnaire
IVD CRO Qualification Questionnaire
Conclusion
Conducting an IVD clinical performance study is a critical step in the development and commercialization of an IVD device. By following best practices, working with experienced professionals, and selecting the right CRO, IVD device manufacturers can generate reliable and meaningful data that can support regulatory approval and the device’s commercial success, ultimately benefiting patients and healthcare providers. Adherence to the IVDR and the ISO 20916 standard can help ensure that the data generated is acceptable for regulatory submission and meets the safety and performance requirements for IVDs.
