 August 9, 2023
August 9, 2023
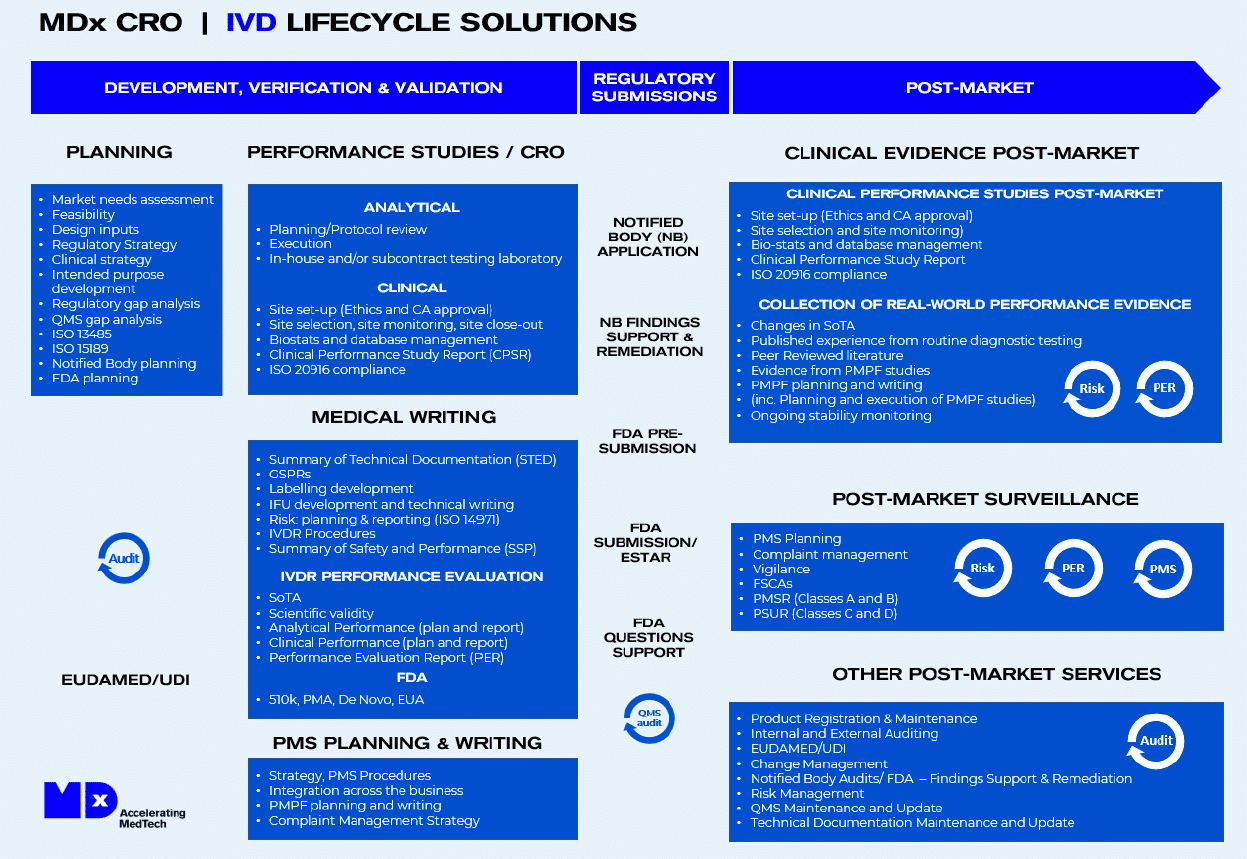
At MDx CRO, our specialized IVD regulatory services cater not only to in vitro diagnostic medical device manufacturers, companion diagnostics (CDx) companies and their pharma partners, laboratories, suppliers, IVD subcontractors and more. Our in-depth knowledge and expertise make us stand out in the constantly evolving landscape of IVD regulations.
Why Choose MDx CRO for IVD Regulatory Services?
- IVD Compliance Mastery: MDx CRO assists IVD manufacturers throughout the entire product life cycle. We ensure you achieve and maintain compliance with IVD regulations across various countries.
- Regulatory Expertise: We not only understand the EU IVDR regulation but are also proficient in the transition from IVDD to IVDR, the FDA IVD requirements in the US, and pre-submission reviews. This comprehensive know-how ensures your product’s smooth and swift entry into the US and EU medical device markets.
- Tailored Services: Be it a start-up, SME, or multinational company, MDx CRO’s bespoke IVD consultancy services are designed to meet your unique needs.
Our IVD Regulatory Service Spectrum
- New Device Development: We guide you through design, development, and market access strategy formulation based on your intended purpose.
- Regulatory Transitions: Navigate the shifts from IVDD to IVDR effortlessly with our expert insights into necessary requirements and adjustments.
- Market Expansion: From the US FDA to EU-IVDR, our regulatory submission support ensures you comprehend and meet the distinct requirements of global markets.
- Commercialization Support: MDx CRO ensures that your device meets all prerequisites for commercial distribution, including device labeling, training materials, and regulatory submissions.
Engage with Seasoned Experts
Our specialists, equipped with industry-centric design and development skills fused with regulatory acumen, are here to provide unparalleled guidance. IVD analytical and clinical performance support can significantly benefit from our specialized expertise.
IVD Manufacturers’ Guide
- IVD Regulatory Strategy: We assist in devising the most fitting regulatory approach for your IVD products with our unique IVD roadmap solutions.
- IVD Technical File Support: Let us help in completing or reviewing your IVD Technical Files to ensure full compliance with the IVDR (EU) 2017/746, paving the way for a hassle-free CE-IVD approval.
- Gap Analysis: Gauge the difference between your Quality Management System and IVDR (EU) 2017/746 requirements to stay ahead.
- Mock & Supplier Audits: Be audit-ready always with our comprehensive audit services, including our IVDR pre-submission service.
Embracing Quality and Excellence
At MDx CRO, quality is our benchmark. Our global team, adept at vendor auditing for diagnostic and laboratory partners, ensures QMS audits in compliance with globally recognized standards.
MDx CRO’s integrated approach, assures sponsors of audit compliance, helping them prepare for any potential international regulatory inspections.
Join forces with MDx CRO and navigate the intricate world of IVD regulations with confidence. Let’s embark on this transformative journey together!
Contact us today to discuss your project needs!
